Understanding how atoms combine to form elements, molecules, and compounds is fundamental to grasping the composition and behavior of matter in all its forms. If you This article explores these foundational concepts, detailing the structures and interactions that define the diversity of material existence from the microscopic to the macroscopic level.
Elements: The Purest Form of Matter
An element is a substance that consists of only one type of atom, each with the same number of protons in its nucleus. The Periodic Table of Elements organizes these substances, currently totaling 118, by their atomic number and chemical properties. Elements are the simplest building blocks of matter and cannot be broken down into simpler substances by chemical means.
Atomic Number: This defines the number of protons in an atom’s nucleus and determines the element’s unique properties. An example is Copper (Cu), who’s atomic number is 29, as there are 29 protons in the nucleus of a Copper atom. Cu can be found in the 4th column down, 11th column from the left, in the Periodic Table (below).

Isotopes: Isotopes are variants of the same element that have the same number of protons but different numbers of neutrons. Isotopes can be stable or radioactive, affecting the element’s atomic mass and nuclear properties. Examples of isotopes of carbon are:
- Carbon-12 (6 protons, 6 neutrons) – normal carbon atom
- Carbon-13 (6 protons, 7 neutrons) – carbon isotope
- Carbon-14 (6 protons, 8 neutrons) – carbon isotope
All of these variations of carbon still have 6 electrons in them when the atoms are in their neutral state, its only the number of neutrons that differs.
Molecules: Bonds Between Atoms
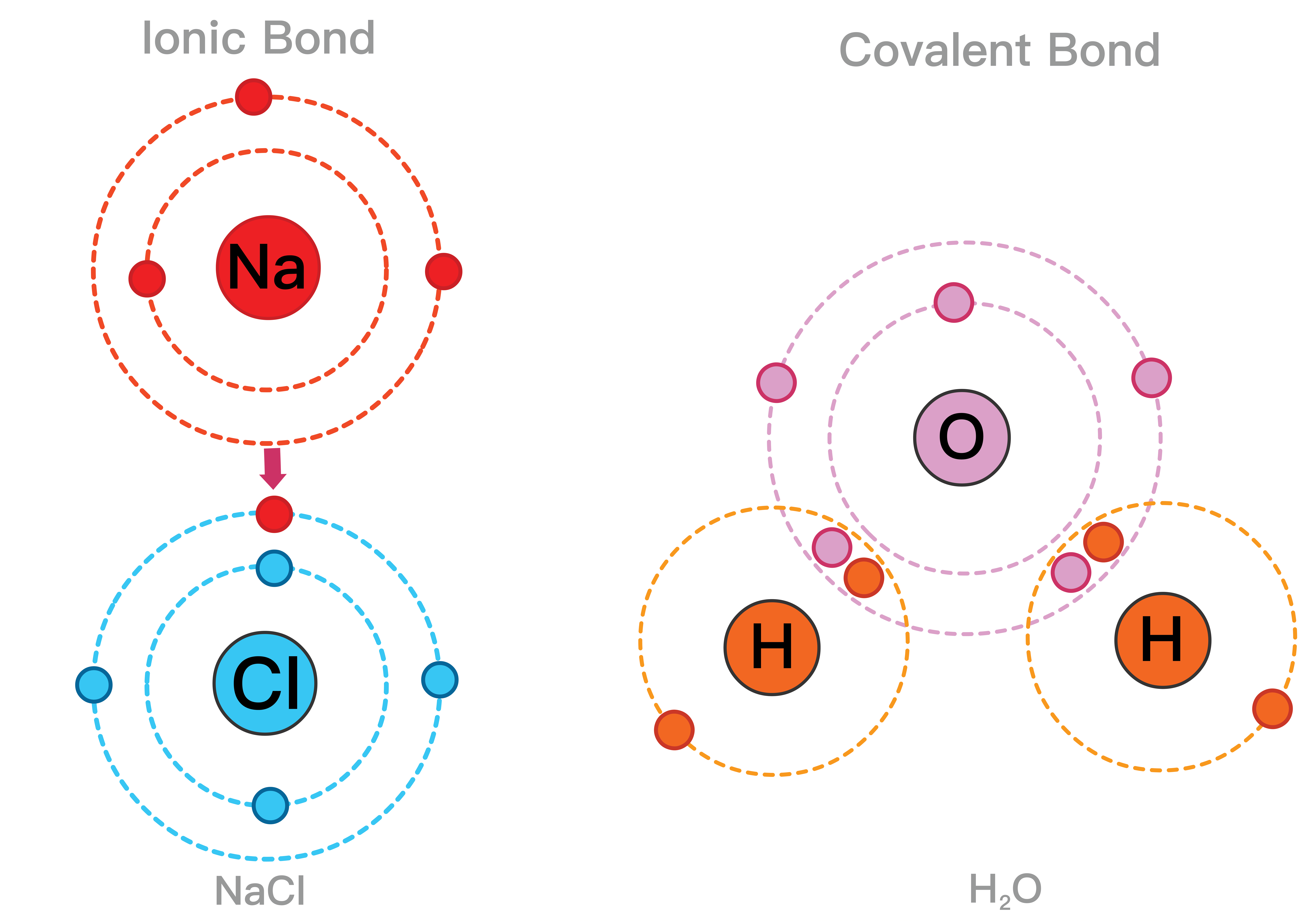
Molecules form when two or more atoms bond together chemically. This bond can involve the same element (such as O2 or N2) or different elements (such as water, H2O). The way atoms bond and the type of molecules they form dictate the properties of the substance. There are many types of bonds that allow matter to coalesce, but the primary three are covalent, ionic, and metallic.
Covalent Bonds
Atoms share one or more pairs of electrons in a covalent bond, usually occurring between nonmetal atoms. The shared electrons stay within the valence layers of their atoms but provide each atom with a more stable electron configuration. Think of covalent bonding as static cling between your shirt and a dryer sheet.
Molecular formulas indicate the number and type of atoms in a molecule, such as H2O for water or CO2 for carbon dioxide. Structural formulas provide a graphical representation of the molecular structure, showing how atoms are bonded.

Ionic Bonds
In an ionic bond, atoms transfer one or more electrons from one atom to another, typically occurring between metal and nonmetal atoms. This transfer results in the formation of positively charged ions (cations) and negatively charged ions (anions). That is to say one of the atoms becomes more positively charged, and the other becomes more negatively charged.
The electrostatic attraction between these oppositely charged ions holds them together in an ionic compound. Think of ionic bonding as the strong attraction between a magnet and a piece of iron, where the magnetic force keeps them firmly connected.
Metallic Bonds
Metalloids bond together into a lattice structure, which is different than liquids and gasses. In a metallic bond, atoms within a metal release some of their electrons, which move freely throughout the entire structure, creating a “sea of electrons.” These delocalized electrons are not bound to any specific atom, allowing them to flow easily and conduct electricity. This electron cloud provides metals with their characteristic properties, such as conductivity, malleability, and ductility. Think of metallic bonding as a community garden where everyone contributes seeds, and the resulting plants are shared and accessible to all, benefiting the entire community.
Compounds: A Combination of Different Elements
A compound is a type of molecule consisting of two or more different elements chemically combined in a fixed ratio. While molecules are mixtures of elements, in general, compounds are specifically those molecules which have different elements mixed to form the molecule. Compounds can be broken down into simpler substances through chemical reactions and have properties that are distinct from those of their component elements.

Ionic Compounds: These form when electrons are transferred from one atom to another, creating ions that are electrically charged. The resulting attraction between oppositely charged ions forms an ionic bond, as seen in sodium chloride (NaCl).
Covalent Compounds: These occur when atoms share electrons, as seen in carbon dioxide (CO2). Unlike ionic compounds, covalent compounds can consist of molecules with definite shapes.
Chemical Reactions: The Path to New Substances
Chemical reactions involve the rearrangement of atoms to form new substances. This process involves breaking old bonds and forming new ones, governed by the laws of conservation of mass and energy.
In a chemical equation, reactants are substances that start a reaction, and products are what result from the reaction. For example, when hydrogen gas (reactant) reacts with oxygen gas (reactant), water is formed (product).
This equation shows (2) Hydrogen molecules (H2 is 2 atoms of hydrogen bonded together as a pair, and there are (2) of them, or (4) total hydrogen atoms) combined with (1) Oxygen molecule (O2 is (2) atoms of oxygen bonded together as a pair). To satisfy the conservation of mass, the number of atoms for each element must be the same on both sides of a chemical equation. The product, therefore, results in (2) H2O molecules, satisfying this as a balanced equation.
Conclusion
The composition and behavior of matter in its various forms result from the intricate interplay of elements, molecules, and compounds. From the building blocks of elements to the complex arrangements of atoms in molecules and compounds, the diverse manifestations of matter emerge from the interactions between these particles. Chemical reactions facilitate the transformation of one substance into another, governed by the laws of conservation of mass and energy. Understanding the structures and bonds that govern the material world not only deepens our scientific knowledge but also enables us to harness the properties of matter for practical applications across numerous fields, shaping our technological advancements and driving innovation.