What State of Matter is Electricity?
Believe it or not this question is asked quite often. Electricity is very difficult to understand for those who aren’t educated about it, so it’s not a dumb question. Many people see lightning and think that is what electricity is – some zappy fluid thing that is made of matter; but electricity actually isn’t matter – nor is it energy. In fact what makes something electric is simply a property of matter, which allows some fundamental particles in an atom to “have charge.” Some particles in matter have charge, while others don’t. This means that some particles are electric, while others are not. Let’s dig a little into the types of matter so we can understand how electricity plays into the equation.
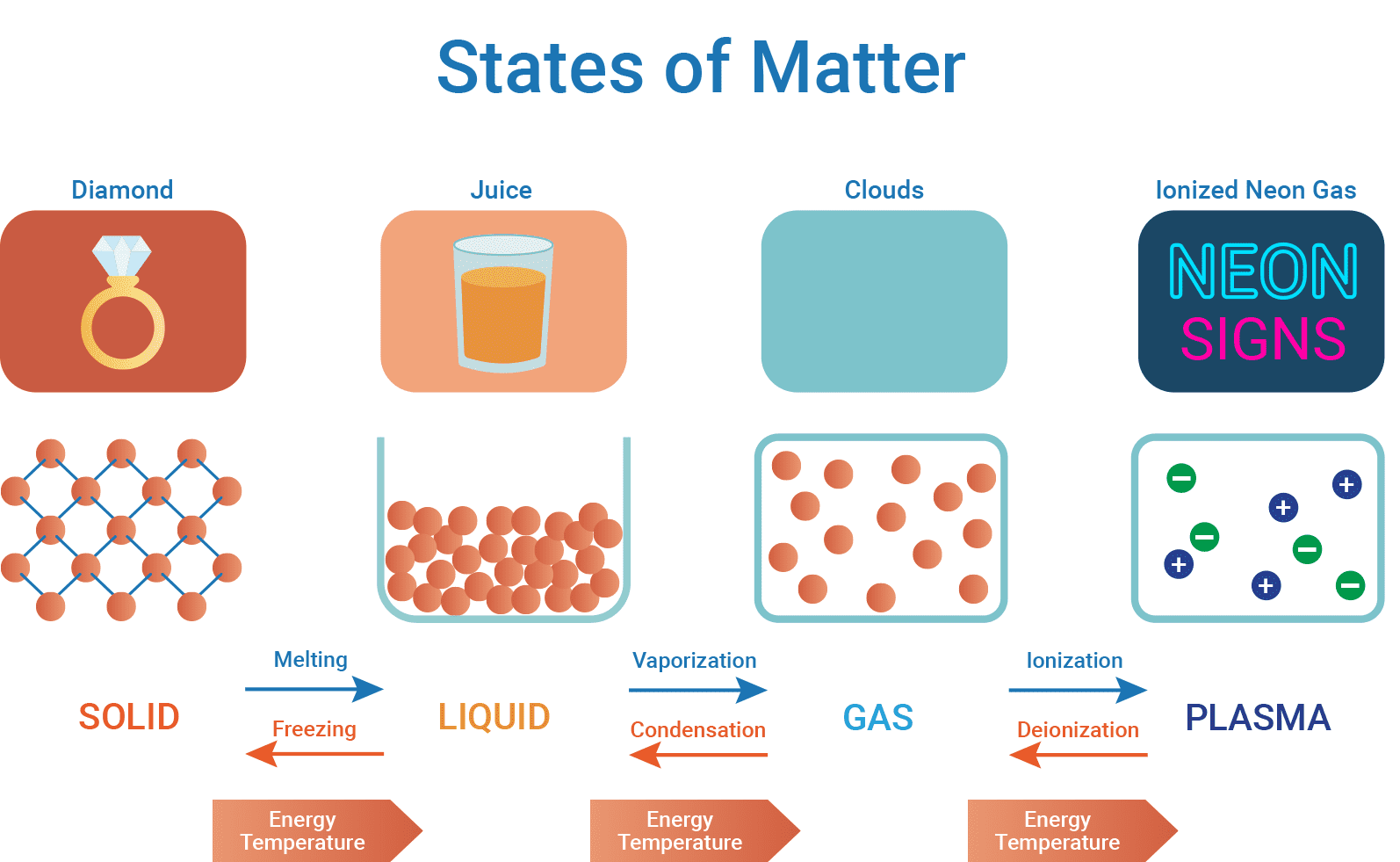
Matter can exist in several distinct states, primarily solid, liquid, gas, and plasma. Each state has unique characteristics based on the arrangement and energy of its particles. Understanding these states provides insights into the fundamental properties of matter and the dynamics of energy flow within and between these states. This discussion will explore the construction and behavior of each state, their interaction with energy, and practical examples. As well as how electricity conducts through different states of matter.
Solids
Solids are characterized by their defined shape and volume. The particles (atoms or molecules) in solids are tightly packed, often in a regular, repeating pattern called a crystal lattice. This close packing is held in fixed positions by strong attractive forces, such as ionic or metallic bonds. This results in solids being incompressible and rigid.

In solids, the particles vibrate about fixed positions because the kinetic energy is low relative to the strong intermolecular forces that bind them. Within solids, thermal and electrical conduction can transfer energy from one particle to another without significant movement of the particles themselves.
In terms of electricity, metals are excellent conductors because they have free electrons (often called conduction electrons) that can move easily throughout the atomic lattice. This movement of electrons under the influence of an electric field constitutes electrical current.
Liquids
Liquids have a fixed volume but no fixed shape, conforming to the shape of their container. The looser arrangement of particles in liquids, compared to solids, enables them to move freely around each other.

In liquids, the particles are still attracted to each other but with weaker forces. This allows them to move more freely and slide past one another. This mobility gives liquids the ability to flow and take the shape of their container while maintaining a relatively constant volume. Particles in liquids, while more loosely organized than in solids, still exert enough attractive forces to keep themselves cohesive.
The kinetic energy in liquids is higher than in solids. Which allows particles to partially overcome intermolecular attractions and move throughout the liquid. Heat transfer in liquids occurs through conduction and convection. With convection involving the movement of groups of molecules within the liquid.
Conductivity in Liquids
Electricity generally does not conduct well through pure liquids unless they contain ions. In pure liquids (e.g., distilled water), there are very few free-moving charge carriers available to conduct electricity efficiently. The molecules in pure liquids are electrically neutral and held together by covalent bonds. Which do not readily allow the free movement of electrons or ions. Ionic solutions, like saltwater, conduct electricity through the movement of ions, not electrons, in response to an electric field.
In contrast, ionic solutions, such as saltwater (sodium chloride dissolved in water), do conduct electricity quite well because they contain mobile ions. When an ionic compound like salt (NaCl) dissolves in water, it dissociates into its component ions (Na+ and Cl-). These positively and negatively charged ions can move freely through the solution in response to an applied electric field. Recall that an ion is an entire atom which has either gained positive or negative overall charge. So rather than using charged particles for conduction, entire charged atoms (ions) are the charge carriers in liquids.
Gases
Gases have neither a fixed volume nor a fixed shape, expanding to fill any container. The particles in gases are very far apart and move randomly and rapidly, making gases compressible. In gases, the spacing between particles are very wide and have minimal attractive forces between them. The particles move randomly and independently, constantly colliding with each other and the container walls. This high degree of particle mobility and lack of strong attractive forces result in gases being able to expand and occupy the entire volume of their container, taking on the shape of the container.

In gases, particles have high kinetic energy. This allows them to move independently of each other, overcoming intermolecular forces almost entirely. Energy transfer in gases primarily occurs through conduction and convection, with the random movement of particles facilitating the spread of energy.
Most gases are poor conductors of electricity because their free electrons are not in sufficient proximity to one another to allow a a steady current to flow. However, if gases become ionized, they turn into plasma and can conduct electricity. For example, lightning is a phenomenon that involves the formation of plasma and the conduction of electricity through ionized air molecules.
Plasma
Plasma, often referred to as the fourth state of matter, consists of a hot, ionized gas with equal numbers of positively charged ions and negatively charged electrons. Plasmas have no fixed shape or volume and are highly electrically conductive. Plasmas can be formed when gases are subjected to extremely high temperatures or strong electromagnetic fields, causing the atoms or molecules to become ionized.

When energy is supplied to a gas, such as through heating or electromagnetic radiation, the atoms or molecules can become excited and lose some of their electrons, resulting in the formation of positively charged ions and free electrons. As more and more atoms or molecules become ionized, the gas transitions into a plasma state. In this state, the positively charged ions and negatively charged electrons are no longer bound together but can move freely, creating a highly conductive and reactive environment.
The energy in plasma is typically so high that electrons are freed from atoms, creating a soup of charged particles. This state allows for the efficient conduction of electricity and is responsive to magnetic and electric fields. Plasmas are in stars, including the sun, and in man-made devices like fluorescent light bulbs and plasma TVs. In these examples, plasma conducts electricity that powers the light-producing reactions or displays.
Transitions Between States
Phase Changes:
Transitions between different states of matter occur through the addition or removal of energy. For example:
- Melting: Solid to liquid by heating
- Freezing: Liquid to solid by cooling
- Vaporization: Liquid to gas by heating
- Condensation: Gas to liquid by cooling
- Ionization: Gas to plasma by heating or applying a strong electromagnetic field

The principles of thermodynamics, especially the conservation of energy, govern these transitions. This principle dictates that the total energy change (heat absorbed or released) during a transition must balance. Under normal conditions, when a substance transitions between different states of matter, it typically follows a sequential path: solid → liquid → gas (or vice versa). However, in certain situations, particularly when the involvement of intense energy is present, a substance can skip one or more of these intermediate states and directly transition from one state to another.
One example is the vaporization of copper during an arc, which is a process that occurs in welding, electrical switches, and other high-energy applications. In this process, the copper metal, which is initially in a solid state, can directly transform into a gaseous state (vapor) without going through the liquid phase.
How Electricity Interacts with the States of Matter
Now that we’ve explored the different states of matter—solid, liquid, gas, and plasma—let’s see how electricity interacts with each of them. It’s important to remember that electricity itself isn’t matter or energy; rather, it’s a phenomenon that occurs when charged particles interact with one-another. These charged particles are typically electrons, which are fundamental components of matter.
Solids
In solids, particularly in metals, electricity can flow relatively easily because metals have free electrons that can move within their structure. This is why metals are great conductors of electricity. In non-metal solids, like wood or rubber, electricity doesn’t flow as freely because the electrons are tightly bound and can’t move as easily, making these materials insulators.
Liquids
In liquids, some substances, like saltwater, allow electricity to flow because they contain charged ions that can move when an electric field is applied. This is why electrolytes, like those in your body or in batteries, are so crucial for electrical conductivity in liquid form. Pure water, on the other hand, is a poor conductor because it lacks free ions, but even the smallest impurities can make it conductive.
Gases
Gases are generally insulators, as the particles are widely spaced and not bound together in a way that allows for the easy movement of electrons. However, when enough energy is added (for example, through a high voltage), gases can become ionized and turn into a plasma, allowing electricity to flow. This is why lightning, which occurs in the atmosphere, is a form of plasma. Plasma is actually a state of matter where gas is energized to the point that electrons are freed from their atoms, allowing for free movement of charged particles, making it an excellent conductor.
Plasma
Finally, plasma is the most electrically active state of matter. In this state, the particles are highly energized and ionized, which means they contain free electrons and positively charged ions. This ionization state of matter allows electrons to be stripped away from atoms, creating a mix of charged particles with intense heat and energy which can be incredibly useful to us. Examples of plasma are lightning, the sun and stars, neon signs, and plasma TVs.
As we can see, electricity is not a state of matter in itself, but rather, a behavior of matter. Certain materials allow the flow of electricity because their particles can either move freely or become ionized, depending on the state they are in. So while electricity isn’t matter, it certainly interacts with matter in all its forms, transferring electrical energy through various materials.
Conclusion
The states of matter—solid, liquid, gas, and plasma—each exhibit unique properties and behaviors, influenced largely by the organization and flow of energy and particles within them. Understanding these states allows scientists and engineers to manipulate matter for a variety of applications, from manufacturing and transportation to energy generation and high-tech industries. The study of how electricity interacts with these states of matter further enhances our ability to harness and utilize this critical form of energy effectively across different mediums. This foundational knowledge not only enriches our understanding of the physical world but also drives innovation in technology and improves the sustainability and efficiency of energy-dependent processes. For more on matter, check out “Principles of Matter”.