The atom, a fundamental building block of matter, has captivated scientists for centuries. Our understanding of the atom has evolved from simple models to complex quantum mechanical descriptions that explain its behavior and interactions. This exploration delves into the structure of the atom, the development of atomic theory from the Bohr model to quantum mechanics, and the fundamental particles that comprise protons and neutrons.
Historical Perspectives on Atomic Structure
The development of atomic theory began with John Dalton in the early 19th century. He proposed that elements consist of indivisible particles or atoms. This theory evolved through the discovery of the electron by J.J. Thomson in 1897, leading to his plum pudding model, which suggested that electrons float within a positively charged substance.
Rutherford’s Nuclear Model
Ernest Rutherford’s gold foil experiment in 1911 revolutionized the atomic model by demonstrating that atoms consist of a dense central nucleus surrounded by electrons. This led to the planetary model, where electrons orbit the nucleus much like planets orbit the sun.

Bohr Model of the Atom
Niels Bohr further refined the atomic model in 1913 by introducing quantum theory to explain the electron’s behavior. Bohr proposed that electrons orbit the nucleus in specific, quantized orbits without radiating energy. According to his model, electrons could only gain or lose energy by jumping from one fixed orbit to another, emitting or absorbing photons in the process. This model successfully explained the hydrogen spectrum but struggled with more complex atoms.

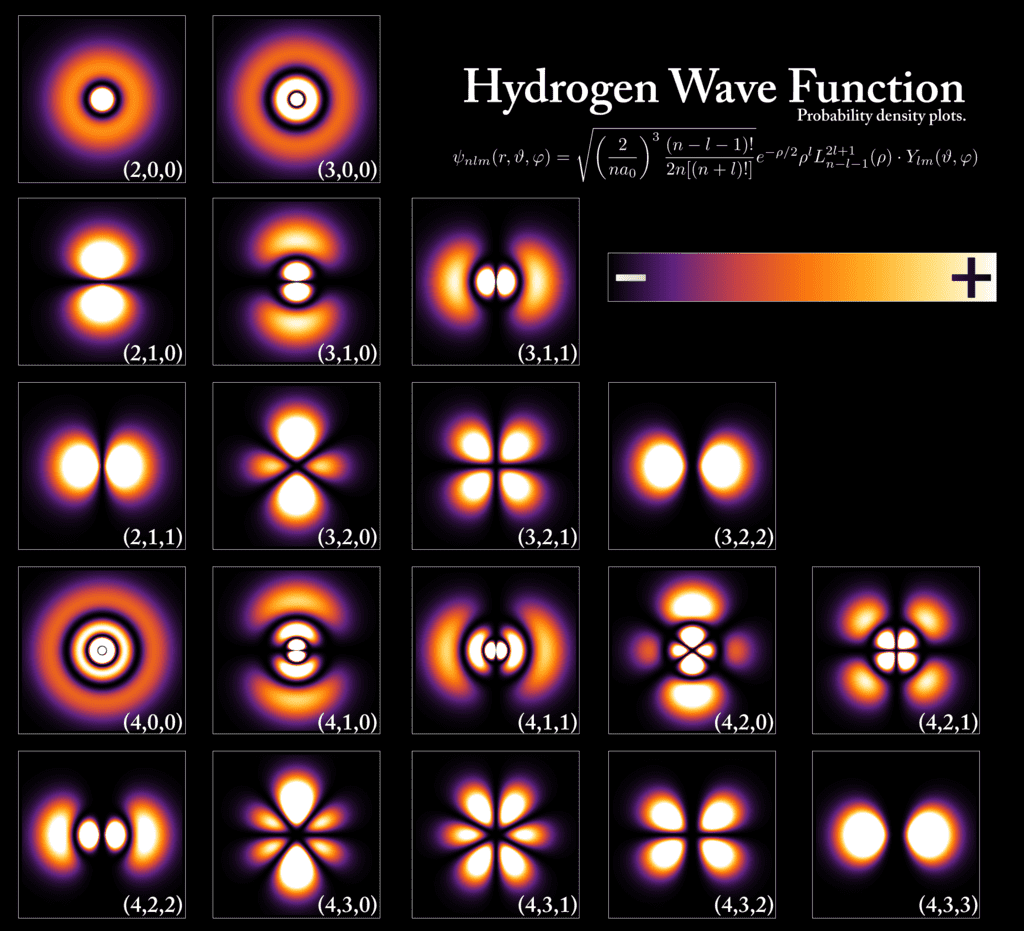
Quantum Mechanics and Electron Orbitals
The limitations of the Bohr model led to the development of modern quantum mechanics. Which provides a more comprehensive and accurate description of atomic structure. Proving that electrons cannot be in constant orbit around a nucleus without losing energy meant that electrons would eventually spiral down into the nucleus if the Bohr model was correct – which is not what is occurring. This meant a new model needed to be developed to explain atoms at a new “quantum” level.

Source – https://socratic.org/
Basic Structure of the Atom
As quantum mechanics understands it, an atom consists of a nucleus surrounded by a cloud of electrons. The nucleus, making up the bulk of an atom’s mass, contains protons and neutrons, collectively known as “nucleons”. Protons carry a positive electric charge, while neutrons are electrically neutral. Electrons, which are negatively charged and have a much smaller mass compared to nucleons, orbit the nucleus in bizarre ways and are responsible for the chemical properties of atoms.

In the quantum mechanical model, orbitals, not fixed orbits, describe electrons. These orbitals are probabilistic distributions indicating where we are most likely to find electrons. By using Schrodinger’s equations to figure out areas electrons are definitely NOT located, we’ve been able to define zones or areas where they MAY be located at any given time. Since we cannot observe electrons directly and they change from energetic to material states when measured, probability remains our best method for making educated guesses about their behavior.
Unlike the well-defined orbits of the Bohr model, orbitals are defined by complex shapes (such as s, p, d, and f orbitals) and are characterized by three quantum numbers:
- Principal Quantum Number (n): Indicates the energy level of the orbital and its relative size.
- Angular Momentum Quantum Number (l): Determines the shape of the orbital.
- Magnetic Quantum Number (m): Specifies the orientation of the orbital in space.
This model accounts for the uncertainty in predicting the exact location of an electron at a given time. This process adheres to the Heisenberg Uncertainty Principle, which states that we cannot simultaneously know the position and momentum of an electron with precision.
Fundamental Particles: Protons and Neutrons
The protons and neutrons that make up the nucleus are, themselves, composed of smaller particles called quarks, held together by gluons, which mediate the strong nuclear force. Quarks are elementary particles that come in six flavors (up, down, charm, strange, top, and bottom). However, only up and down quarks have evolvement in the composition of protons and neutrons:
- Protons: Consist of two up quarks and one down quark.
- Neutrons: Consist of one up quark and two down quarks.
The theory of quantum chromodynamics (QCD) describes the interaction between quarks and gluons. QCD explains how the strong force confines quarks within protons and neutrons, preventing their isolation.
String theory is one of several theories which attempts to go further down the rabbit hole of hypothesizing what composes quarks. String theorists believe there may be vibrating one-dimensional “strings” that give form to quarks and electrons. This, however, is not the only theory in existence, just the most popular.

Outside the nucleus, electrons exist as elementary particles, not composed of smaller particles. While nucleons stay bound within the nucleus of atoms, electrons move about the outer region of the atom. Electrons are not only fundamental to atomic structure but also play crucial roles in various physical phenomena:
- Electrical Charge and Current: Electrons carry a negative charge and are the primary carriers of electric current in conductors. When we apply voltage across a conductor, electrons move, creating an electric current. This movement is fundamental to almost all electronic devices and electrical systems.
- Chemical Properties: Electrons are key players in chemical reactions, particularly through the formation and breaking of chemical bonds. The arrangement of electrons in the outer shells of atoms determines the chemical properties of an element, including its reactivity and how it bonds with other atoms.
- Quantum Mechanics: Electrons exhibit wave-particle duality, a fundamental concept in quantum mechanics. This means they exhibit properties of both particles and waves. Understanding electron behavior on a quantum level is crucial for developing technologies such as semiconductors and quantum computers.
- Magnetic Properties: The magnetic properties of materials are largely due to the behavior of electrons. Especially their spins and orbital movement around the nucleus. For instance, the alignment of electron spins in the same direction can lead to ferromagnetism, as seen in iron.
- Optical Properties: Electrons are responsible for the absorption and emission of light in materials. When electrons absorb energy, they can jump to higher energy levels. When they fall back to lower energy levels, they emit light. This principle is the basis for technologies like lasers and fluorescent lighting.
- Thermal Conductivity: In metals, electrons also contribute to thermal conductivity. The same free electrons that conduct electrical current also carry thermal energy through the metal.
Energy Levels and Orbitals
Electrons exist within areas called “energy levels,” or “electron shells” and can move up or down to different energy levels when they either absorb or release energy, respectively. The number of electrons which may exist within an energy level varies. Each energy level contains defined regions known as orbitals. Energy levels help us determine how much energy an electron in the area has. While orbitals help us to nail down a relative probabilistic location of an electron, within this energy level.
Energy Levels
Energy levels, also known as electron shells, refer to the broad, defined layers surrounding an atom’s nucleus where electrons reside. These levels indicate the potential energy of electrons and are quantized, meaning electrons at a given level possess a specific amount of energy and no other. The energy levels are typically indexed by principal quantum numbers n=1, n=2, n=3, etc. Higher energy levels are farther from the nucleus and can accommodate more electrons.

Orbitals
Within each energy level, electrons do not simply float randomly. Instead, they reside in specific regions called orbitals. Orbitals describe the spatial distribution of an electron’s probability density — that is, where an electron is likely to be found at any given time. Each orbital has a unique shape and orientation in space, which can be spherically symmetric (s orbitals), dumbbell-shaped (p orbitals), clover-shaped (d orbitals), or even more complex (f orbitals).

Source- https://en.wikipedia.org/
Key Differences:
- Conceptual Nature: Energy levels are more abstract, indicating the energy an electron possesses without specifying its location. Orbitals provide a more detailed description, defining the shape and volume in space where electrons are likely to be found.
- Capacity and Arrangement: A single energy level can contain multiple orbitals. For instance, the first energy level contains only one s orbital, the second level contains one s orbital and three p orbitals, and higher levels include d and f orbitals in addition to s and p orbitals.
- Quantum Numbers: The description of orbitals involves more quantum numbers than energy levels. While the principal quantum number 𝑛 determines the energy level, the azimuthal quantum number 𝑙, magnetic quantum number 𝑚, and spin quantum number 𝑠 are essential for defining an orbital within that level.
Despite their differences energy levels and orbitals are interconnected. The energy level sets the stage, providing a layer-like structure, while orbitals detail the specific environments within these layers where electrons operate. This interplay is crucial for understanding electron configurations, chemical bonding, and the chemical properties of elements.
Energy Levels and Spectra
The energy levels of electrons in atoms determine the spectral lines emitted or absorbed. Each element has a unique atomic spectrum, which can be used for identification. These spectra result from electrons transitioning between energy levels, releasing or absorbing quantized amounts of energy (photons) corresponding to specific wavelengths of light. In short, the colors we see are from photons bouncing back to our eyes after being emitted from electrons changing energy levels. Without this, we would not see color.

Conclusion
The atom is a marvel of complexity and simplicity, governed by principles that stretch our understanding of the natural world. From Bohr’s quantized orbits to the probabilistic orbitals of quantum mechanics, the journey through atomic theory reflects broader shifts in scientific thinking and technological capability. Understanding the atom is crucial not only for chemistry and physics but also for innovations in materials science, electronics, and quantum computing. By exploring the intricate details of atomic structure and the fundamental particles within, we gain a deeper appreciation for the fundamental constituents of matter and begin to build a concept in our minds for how electrical current traverses matter.